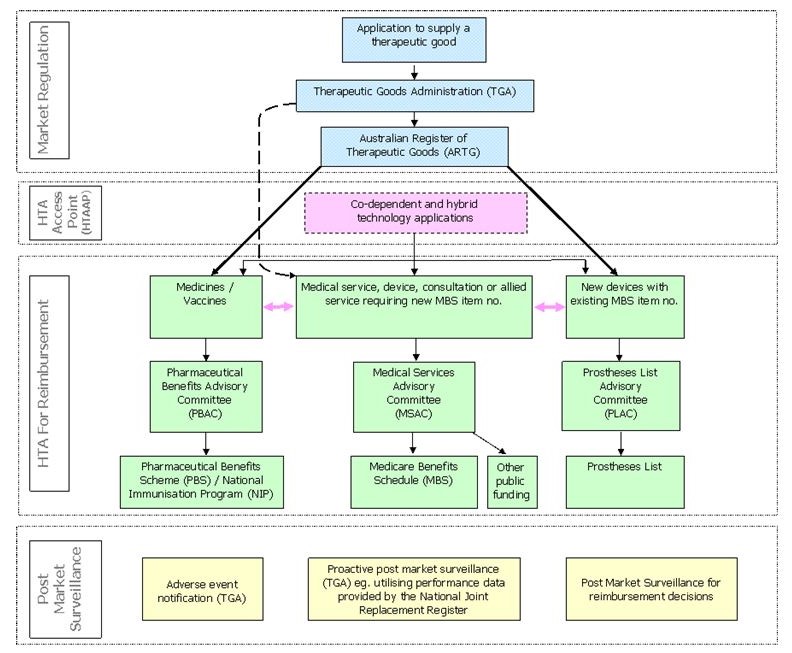
Australian Government Health Technology Assessment Processes
The
Therapeutic Goods Administration (TGA), the
Medical Services Advisory Committee (MSAC), the
Pharmaceutical Benefits Advisory Committee (PBAC) and the
Prostheses List Advisory Committee (PLAC - formerly the Prostheses and Devices Committee), each perform health technology assessment (HTA) processes to provide advice to the Australian Government Department of Health.
Each entity has complex and inter-dependent relationships with discrete functions that respond to different policy needs.
Efficient and effective HTA processes are crucial to supporting sustainable management of subsidised health technologies. Consistent application of evidence across Australian Government HTA processes is an important element in ensuring stakeholder confidence in the HTA framework by creating certainty in how these processes are implemented and their achieved outcome.
Each HTA agency supports the Australian Government HTA framework through the following functions:
Assessment of the safety and efficacy of health technologies for market regulation to ensure that therapeutic goods are safe, perform as intended and are produced using appropriate quality controls before marketing approval is granted in Australia through the Australian Register of Therapeutic Goods (ARTG); and
Appraisal of the comparative safety, clinical and cost effectiveness of health technologies which informs decisions about:
Public funding of medical services (with or without a device), procedures and diagnostic technologies, pharmaceuticals and vaccines through the Medicare Benefits Schedule (MBS), the Pharmaceutical Benefits Scheme (PBS) and the National Immunisation Program (NIP) respectively;
Private health insurance reimbursement of prosthetic devices through the Prostheses List; and
Post market surveillance of these health care interventions to inform ongoing decisions about the marketing approval of therapeutic goods or the reimbursement of health technologies that prove not to be safe or do not perform as intended.
Below is a map of current Australian Government HTA processes for market entry and for reimbursement processes.
.